ECHA’s Restriction Proposal for the Ban of Microplastics
19 Mar 2021
Intertek, a leading Total Quality Assurance provider to industries worldwide, is pleased to announce it is prepared to support cosmetic companies in understanding and preparing for the European Union (EU) proposed microplastics restriction to ensure successful market entry into the EU.
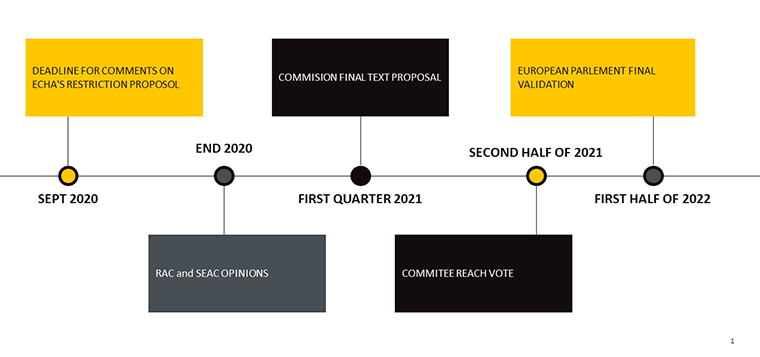
As part of its plastics strategy and sustainable development goals, the European Commission (EC) asked the European Chemicals Agency (ECHA) to complete a restrictions proposal (Annex XV report) for intentionally-added microplastics used in products that are placed on the European Union/European Economic Area (EU/EEA) market. The proposal aims to ban microplastics in products such as cosmetics, detergents, fertilisers and more. According to ECHA, the ban of microplastics would prevent the release of 500,000 tonnes of microplastics into the environment over a 20-year period.
The EC’s proposal to amend the list of substance restrictions in Annex XVII of the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) Regulation will be put to a vote before the EU Member States in the REACH Committee in the second half of 2021. If approved, the restrictions will impact cosmetic manufacturers.
What are microplastics?
A microplastic can be defined as a solid polymer containing particles with dimensions between 100 nm and 5 mm, to which additives or other substances may have been added. This definition does not apply to:
- Polymers in nature that have not been chemically modified;
- Biodegradable polymers; or
- Polymers with water-solubility >2 g/L.
Substances and mixtures categorised as microplastics can be placed on the market in the following scenarios:
- If the polymer loses its microplastic physical properties permanently during the final product formulation (i.e., melted polymer).
- Annual reporting to ECHA is required (EIF +36 months)
- If the polymer loses its microplastic physical properties permanently during the use of the final product (i.e., film forming on the skin).
- Annual reporting to ECHA is required (EIF +36 months) and labelling (EIF +24 months)
Who is impacted?
The EU microplastic restriction will impact cosmetic manufacturers, as they will need to comply by the transition periods outlined below:
- Rinse-off products: EIF +4 years
- Leave-on products (except makeup products, nail polish, lipstick): EIF +6 years
- Make up products, nail polish, lipstick*: EIF +8 to 12 years
*The microplastic commission is discussing the implementation of a longer transition period, as these products have a lower environmental impact.
Key deadlines prior to the published microplastics restriction:
To ensure successful market entry into the EU, cosmetic businesses should be aware of and prepared for the microplastics restriction to ensure their cosmetic product formulas are compliant.
Intertek Solutions
Intertek provides a comprehensive range of services for beauty and personal care products to ensure quality, safety and efficacy, in addition to helping companies understand and comply with regulations around the world. With its comprehensive service offering, Intertek is uniquely positioned to ensure companies understand changes in regulation and meet the necessary requirements to continue importing products to the EU.
Our services include:
- Toxicological Safety Assessments
- Toxicological Profiles of Ingredients
- Registration & Notification of New Cosmetic Ingredients
- Registration & Notification of Domestic/Imported Cosmetic Products
- Labelling Reviews
- Literature Review & Data Collection
- Regulatory Dossiers
- Microbiology & Stability Testing
- Cosmetic Packaging Analysis
- Causality Assessment
- Compliance Solutions
- Regulatory Support
- Sustainability Solutions
Our team of experts are available to address the regulatory and compliance needs of the cosmetics, home and personal care industry. If you have any questions or concerns, we’re here to help.
For more information about Intertek’s cosmetic services, please visit: www.intertek.com/beauty-personal-care/
For Media Information
Please contact:
Tracy Veale
Global Marketing and Communications Director
T: +1 905 542 2900
E: tracy.veale@intertek.com
For Technical Information
Please contact:
Morgane Bily
Regulatory Technical Team Manager
T: +33 (0)1 83 75 80 90
E: morgane.bily@intertek.com
About Intertek
Intertek is a leading Total Quality Assurance provider to industries worldwide. Our network of more than 1,000 laboratories and offices in more than 100 countries, delivers innovative and bespoke Assurance, Testing, Inspection and Certification solutions for our customers' operations and supply chains. Intertek Total Quality Assurance expertise, delivered consistently with precision, pace and passion, enabling our customers to power ahead safely.