Solutions to meet state-of-the-art standards on safety and electromagnetic compatibility (EMC), including IEC 60601-1, 60601-1-2, Home Healthcare, medical imaging equipment, IEC 61010, Implantable medical devices, SPE-3000 and more
Intertek provides expert safety testing and electromagnetic compatibility (EMC) solutions, helping medical device manufacturers meet critical global standards with greater efficiency, speed, and confidence. From design to market entry, our services ensure compliance with IEC 60601-1, IEC 60601-1-2, and other key regulations for safety, EMC, and medical device performance.
With expertise in medical imaging technologies, implantable devices, home healthcare equipment IEC 61010 for laboratory equipment and much more, we address the unique challenges of modern medical innovations. Our advanced facilities and experienced engineers provide reliable testing that ensures your devices meet the highest standards of safety, performance, and reliability.
Intertek helps you navigate complex compliance requirements and accelerate time to market, delivering confidence that your devices perform safely in real-world environments. We are your trusted partner to bring innovative, compliant, and high quality medical solutions to the global healthcare market.
Overview of IEC 60601-1 Standards and References
End-to-end solutions from product development and risk management file reviews to comprehensive testing to the IEC 60601-1 series.
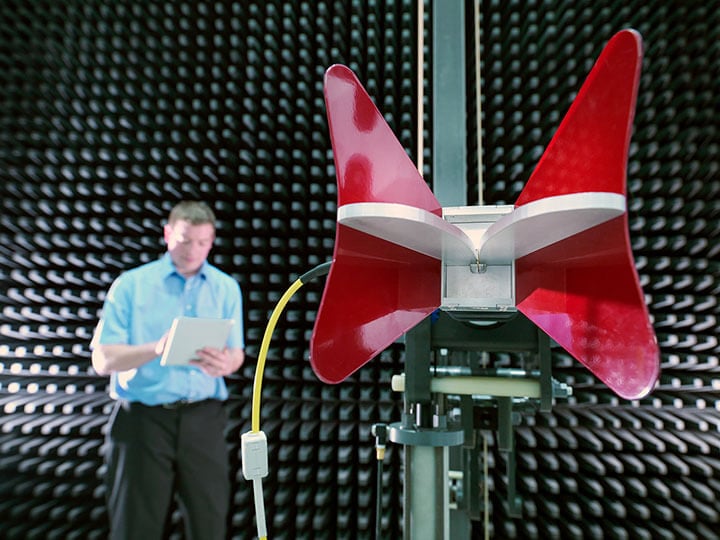
IEC 60601-1-2: Medical Device EMC Testing
IEC 60601-1-2 Electromagnetic Compatibility (EMC) Testing for Medical Devices including IEC 60601-1-2 4th Edition Amendment 2.
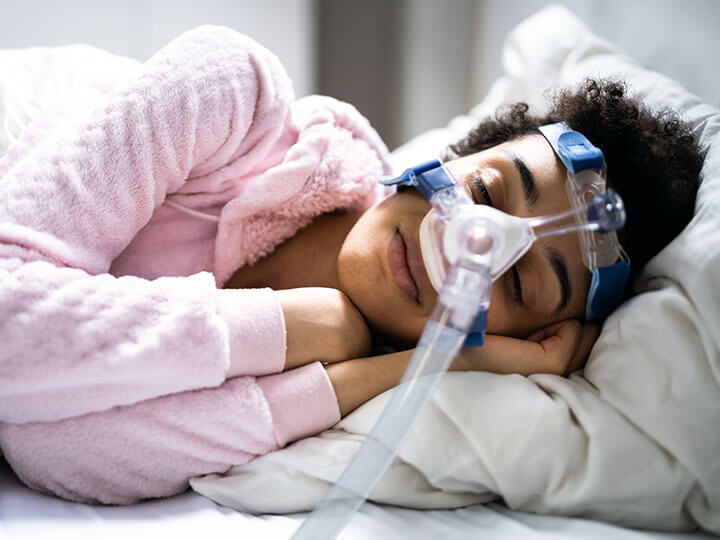
Home Healthcare Equipment Testing and Certification
Comprehensive Testing and Certification solutions for Home Healthcare Equipment to all applicable standards including IEC 60601-1-11.
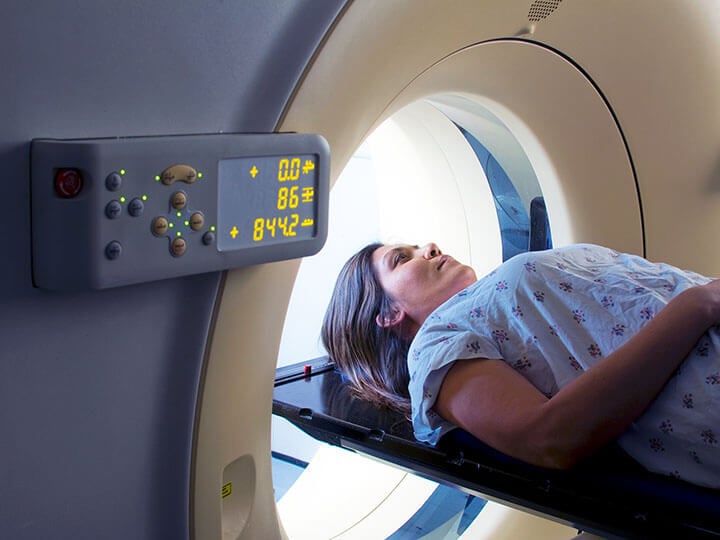
Medical Imaging Equipment Testing
Expert Medical Imaging Equipment Testing and Certification solutions ensuring your medical imaging equipment meets all applicable safety requirements.
Biocompatibility Testing and Evaluation
Unrivaled capabilities in Biocompatibility Testing and Evaluation for medical devices, delivering comprehensive solutions aligned with ISO standards to ensure safety and compliance.
ISO 18562 and VOC Testing for Medical Devices
The industry leader in ISO 18562 and VOC Testing and Evaluation solutions of breathing gas pathways in medical devices with comprehensive expertise and solutions.
IEC 61010: Safety Requirements for Electrical Equipment for Measurement, Control, and Laboratory Use
Critical information on IEC 61010, the standard for testing Medical Laboratory Equipment, to ensure your products remain in compliance with the latest revision of IEC 61010.
Implantable Medical Devices Testing Solutions
Guidance on innovative Implantable Medical Devices such as Cochlear Implants, monitoring devices, pacemakers, etc. to comply with safety, EMC and product-specific standards.
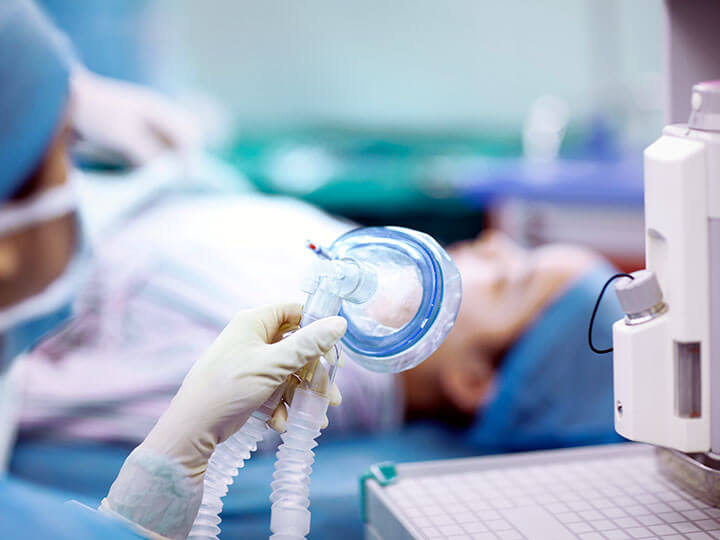
Ventilator Production & Testing
Immediate Ventilator Testing assistance for manufacturers along with regulatory requirement guidance for safety, performance, labeling and more.
SPE-3000: Medical Field Evaluations
For medical products entering the Canadian market, SPE-3000 serves as the model code for the field evaluation of medical electrical equipment (MEE) and medical electrical systems (MES), specifically pertaining to safety from electric shock, fire and mechanical hazards.
SATELLITE™ Client Test Program For Medical Devices
Product compliance testing in your own labs and on your own schedule while obtaining our market-leading Certifications.
Preliminary Design Reviews For Medical Devices
A Preliminary Design Review (PDR) enables you to design with confidence and streamline compliance for your medical device.

Knowledge Center
Download the latest information from our medical device compliance experts.
IEC 81001-5-1 and Cybersecurity for Medical Devices Webinar
Six Compliance-Related Questions About Connected Home Healthcare Devices
Accelerated Stress Testing for Medical Devices
Machine Learning and Artificial Intelligence (AI) in Medical Devices: Webinar | Fact Sheet
ENERGY STAR® Requirements for Medical Imaging Equipment
Creation of IEC 60601-1 4th Edition
IEC 60601-1-2 Ed. 4.1 Overview of Requirements
Medical OEM Wireless Coexistence Testing
Biocompatibility Risk Assessment and Evaluation Plans
For more expert papers, recordings, and presentations, visit our Medical Resources hub.

Follow Us For More!
We’re always adding new content and looking for ways to help you simplify the regulatory and compliance process for medical devices.
*The Intertek legal entities that provide medical device management system certification services (including ISO 13485 and MDSAP) and Notified Body services (MDR 2017/745 and MDD 93/42/EEC) do not provide any consulting services. Clients who have used other Intertek legal entities’ consulting services are not eligible to receive management system certification services or Notified Body services from Intertek.